
Silver Alginate Dressing
Made of natural fibers, has broad spectrum antibacterial.
LUOFUCON® Silver Alginate Dressing is composed of calcium alginate which derived from seaweed through a series of special process and silver particles. It is designed to be highly and fast absorbent. The dressing absorbs exudates and forms a gel-like covering over the wound, maintains a moist environment for wound healing, which also keeps the dressing from adhering to the wound. The most fascinating aspect is the dressing can restrain the bacteria growing and reduce infection.


● Figure1 shows that LUOFUCON® Silver Alginate Dressing leaches about 57% of the total silver during the first 24 hours, and 30%, 24% of its total silver during 24-48, 48-72 hours, respectively. The release profiles indicates that the release of silver from the dressing is well controlled.
Note: Two pieces of the dressing in the size of 10 x 10cm were used in this test. The test was performed by China National Analytical Center at Guangzhou.


Antibacterial Efficacy
● One representative Gram-positive bacteria (Staphylococcus aureus) and one representative Gram-negative bacteria (Pseudomonas aeruginosa) were tested under AATCC 100-2004.
Note: Comparison 1 and 2 are other silver alginate dressings in the market. The test was performed by our own laboratory.
● Figure 2 and Figure 3 show LUOFUCON® Silver Alginate Dressing has four log reduction (99.99%) for both organisms, the other two products only have three log reduction (99.9%).
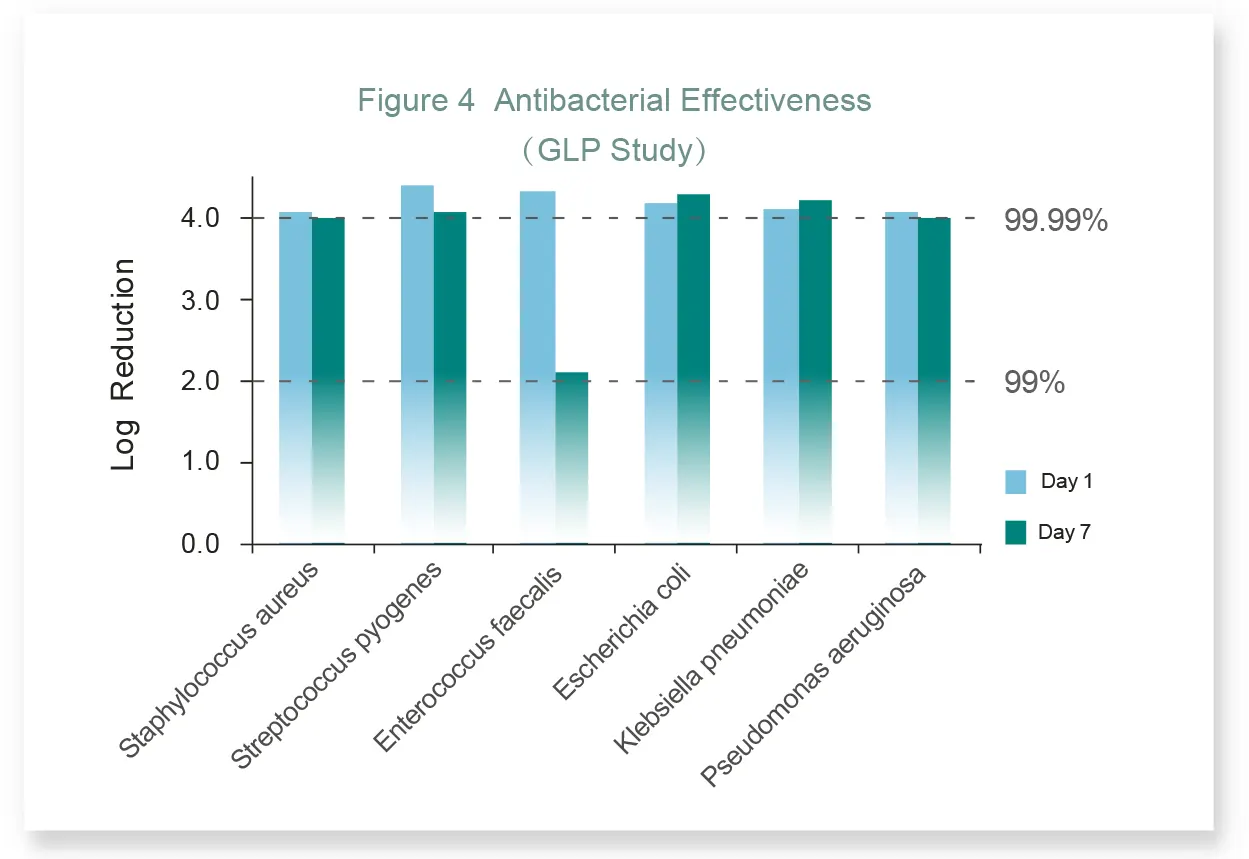
● At day-1, results show LUOFUCON® Silver Alginate Dressing has bacteria reduction of at least four log reduction for all six test organisms;
● At day-7, bacteria reduction for all the organisms is at least two log reduction.
Note: The test was performed under Good Laboratory Practice regulations, by WuXi AppTec, Inc.(Address :1265 Kennestone Circle, Marietta, GA 30066).

Rx Indications
Indicated for moderately to heavily exuding partial to full thickness wounds, including:
- Trauma wounds
- Pressure ulcers
- Diabetic ulcers
- Leg ulcers
- Graft and donor sites
- Post-operative surgical wounds
OTC Indications
Indicated for first aid to help in minor abrasions, minor cuts, lacerations, scrapes, minor scalds and burns.
Contraindications
Do not use LUOFUCON® Silver Alginate Dressing on patients with known sensitivity to silver.
LUOFUCON® Silver Alginate Dressing is not intended for use on third-degree burns.

Tips for using Silver Dressing
- Conduct a comprehensive assessment of the patient, wound and environment before deciding whether a silver dressing is appropriate;
- Document the rationale for using a silver dressing in the patient's healthcare records;
- Choose the silver dressing on the basis of patient and wound needs, ie exudate level, wound depth, need for conformability, odour control, ease of removal and safety;
- For infected wounds, initial use should be for a two week challenge;
- Continued use of silver dressings should include regular review;
- Use silver dressings in the context of a wound management protocol that includes wound bed preparation as appropriate for the wound type;
- Follow manufacturer's instructions regarding indications, contraindications, method of application, wound cleansing procedures, need for dressing moistening before application, and use in patients undergoing MRI or radiotherapy;
- Use silver dressings with caution in children and very large wounds.
Reference: Appropriate Use of Silver Dressing in Wounds, Wounds International, Wounds Internatioal Enterprise House, 2012
Qualification certificate
Instructions for use

Model specifications


References
Related products
A statement on the pictures and data of this article
All the pictures, forms and data in this page are all derived from FORYOU MEDICAL and third party organizations. If you have any questions about the content, you can contact us directly.




